Targeting transformational impact through flexible and adaptable therapy
Our aspiration is to cure solid tumors with a precision, off-the-shelf ‘Standard Pharmaceutical like Cell Therapy’ platform, which can be easily tailored to the needs of individual patients and adapted to multiple disease targets.
Utilizing a deep understanding of cancer biology and the tumor microenvironment, we are developing a flexible and versatile platform to direct the body’s immune cells to target single or multiple tumor antigens to fuel a stronger immune response, while avoiding immune rejection.
By combining our ACCEL (Advanced Cellular Control through Engineered Ligands) technology and Universal Cells’ Universal Donor Cells (UDC) technology, we aim to create a unique platform that offers greater speed and flexibility compared to currently approved chimeric antigen receptor cell therapies and expands potential value for as many cancer patients as possible.
As an Astellas company, Xyphos is the home of the company’s Center of Excellence for Cancer Cell Therapy, uniting expert teams and leading capabilities from across Astellas to build a competitive cancer cell therapy platform with the potential to transform the lives of people with cancer around the world. At Astellas, immuno-oncology is a key area of focus with the goal of delivering therapies to tackle hard-to-treat cancers where few therapies exist.
Watch our video to learn more about our approach to Immuno-Oncology.
ConvertibleCAR™ – Our unique, flexible cell therapy technology
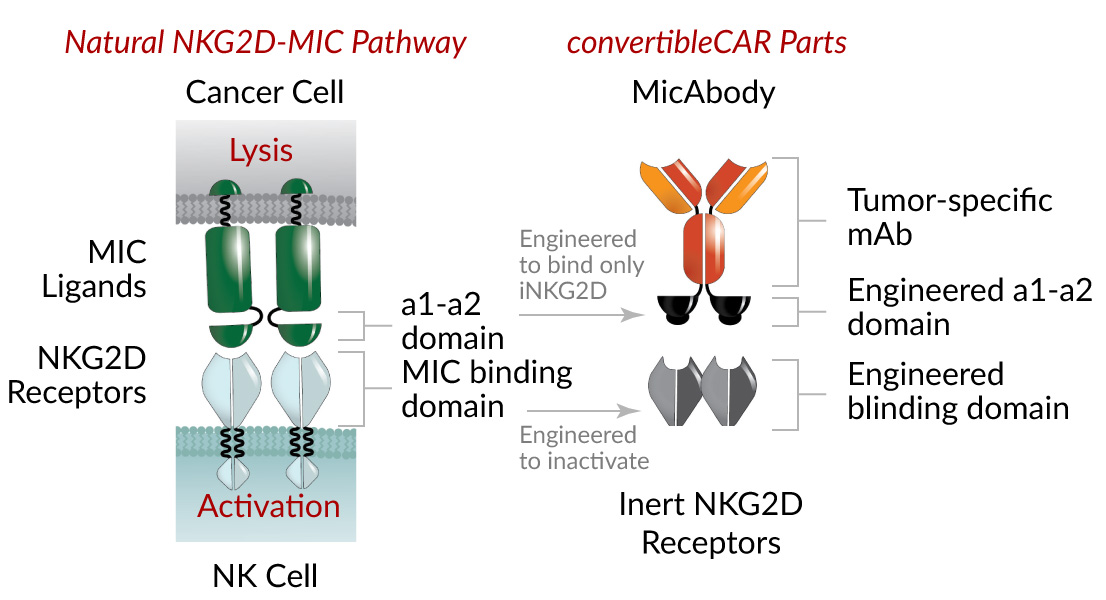
Xyphos’ proprietary convertibleCAR™ (Chimeric Antigen Receptor) technology creates flexible cell therapies that can be engineered and re-engineered inside the body to target more than one tumor antigen, offering improved ways to mobilize immune cells to find and destroy target tumor cells.
ConvertibleCAR™ technology modifies a natural human receptor, NKG2D, which facilitates immune surveillance and exists on certain immune cells – natural killer cells and some T-cells. By utilizing a modified NKG2D receptor, its activity can be controlled through engineered molecules (e.g. bispecific antibodies) that specifically recognize antigens, or binding sites, on tumor cells, in addition to activating the modified NKG2D receptor on immune cells. This enables specific targeting of tumor cells, by bringing them into close proximity with immune cells, which are then activated to attack and destroy them.
Importantly, by attaching various functional molecules, the convertibleCAR™ therapy can be adapted to target different tumor antigens, even as they change to evade detection by the immune system, offering the potential to overcome the challenges of tumor resistance.
Universal Donor Cells – A cutting-edge allogeneic cell therapy platform
Pluripotent stem cells, the foundation cells from which all the body’s cells are made, can be used to produce virtually any therapeutic cell type. Astellas-owned Universal Cell’s proprietary Universal Donor Cells (UDC) technology incorporates multiple cutting-edge approaches to precisely engineer induced pluripotent stem cells, creating a sophisticated, allogeneic cell therapy platform.
A major advantage of UDCs is that a single-cell line can be differentiated into multiple potential cell therapies. This proprietary approach combines nuclease-free gene editing with viral vectors, engineering of immortal stem cells that can form any cell type and specific human leukocyte antigen modifications, to develop cancer cell therapies that can both avoid immune rejection and be used in any patient.